An Update on Treatment of Infantile Hemangiomas
Prevalence of infantile hemangiomas (IHs)
The most common benign tumor that may be found on the head or neck of a child is an IH. These vascular neoplasms are seen more often in female versus male infants, and prematurity or low birth weight (as a result of multiple gestation) are significant predisposing risk factors. Most IHs follow the course of neonatal proliferation, stabilization in late infancy and resolution over a period of years. However, a noteworthy percentage of hemangiomas occur in locations where proliferation is problematic, for example, eventuating in ulceration, structural deformity or other forms of significant morbidity.
Some IHs are associated with internal abnormalities which intensify their degree of severity. For example, the well-described PHACES syndrome features large segmental, usually telangiectatic/minimal change IHs, along with one or more systemic abnormalities: Disorders of cerebral or cervical vascular architecture, atypical cerebral configuration, cardiac structural or functional anomaly, ophthalmologic or endocrinologic dysfunction, and truncal skin or skeletal aberrations.
IHs involving facial segment 3, also known as the 'beard' area, may be accompanied by subglottic hemangiomas which threaten respiration. Periocular IHs have the potential to temporarily or permanently distort development of the visual axis, and the discomfort associated with a perioral or perineal ulcerated hemangiomas confers interminable distress to a growing infant. These complicated IHs require either medical or surgical intervention. Given that the latter option carries risk for permanent scarring, medical treatment is preferred where feasible.
Treatment of IH
Since the fortuitous discovery of oral propranolol for the treatment of IHs, there has been an explosion of literature describing the drug's efficacy, limitations, dosing recommendations, and alternatives. Seeing a formerly inconsolable infant with a large ulcerated IH cooing contentedly in their mother's arms 4 weeks after initiation of therapy is a blessing on both sides of the therapeutic relationship (see Figure 1 for a demonstration of propranolol's efficacy). There has been such great momentum behind this form of therapy that an FDA-approved medical indication for the drug was sought and approved in the spring of 2014.
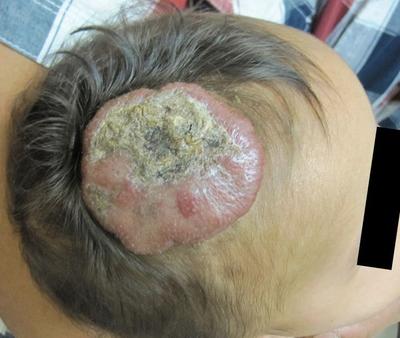
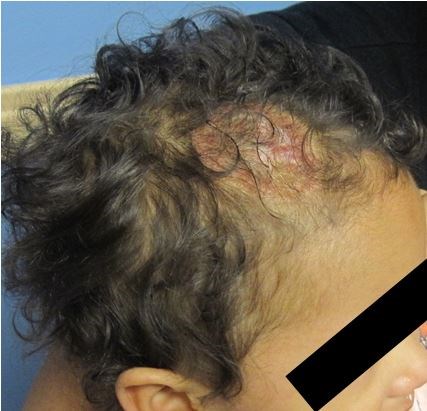
Given the invested involvement of the group making the original discovery and a wealth of pediatric dermatology experts, the new medication, propranolol hydrochloride (4.28 mg/mL), is now available with laudable attributes. The medication does not carry the requirement for hospitalization in healthy infants, and is formulated in a vehicle which averts the noted side effects of dental caries and allergic sensitization to dyes and preservatives. It can also be readily obtained via a number routes that are convenient for the patient's parents/carer.
Given the sheer number of cases for which treatment is indicated, this formulation of the drug may allow physicians in the community to initiate treatment as early as possible, rather than waiting for a referral to a sub-specialist in a tertiary referral center, while the hemangiomas grows. We now know that proliferation begins as early as the second week of life and the major portion of growth may take place within 2-3 months. Reproducibly, in the absence of complication, IHs respond more quickly and completely to treatment initiated early in the proliferative phase, with lower risk of rebound. However, treatment for an entire year is still recommended as early withdrawal of the drug-induced growth suppression can result in the reappearance of IHs.
Adverse events
The side effects associated with propranolol include dental caries, hypotension, insomnia, agitation, aggravation of bronchitis/bronchospasm, gastrointestinal disturbance, dry skin, glucose abnormality, bradycardia, fatigue, and cold hands and feet. The medication is relatively contraindicated in patients with a history of asthma, PHACES, or decompensated cardiac failure. Blood pressure and heart rate monitoring are recommended upon initiation and with dose changes. In fact, a recent study of patients hospitalized for propranolol initiation showed that with frequent assessment following administration, the decreases in diastolic blood pressure and heart rate were not dramatic in most cases.
Alternative treatment options for IHs
Alternatives to systemic therapy with propranolol included topical administration of timolol (see Table 1 for a comparison). The 0.5% gel-forming solution may carry a lower risk of systemic absorption, and when administered as 1-2 drops twice daily, there is no requirement for systemic monitoring. Application of this beta blocker on ulcerated IHs and at mucosal sites, increases risk for access to the circulation, conferring the same risk profile as oral propranolol use. For thin or superficial IHs, the response can be quite significant, albeit at a slower rate than with oral propranolol. This medication is useful for patients in whom oral propranolol is contraindicated or cases where the IHs is less severe (see Table 2 for IH treatment indications [oral propranolol versus topical timolol]).
Table 1. Comparison between propranolol and timolol treatment for IHs
| Propranolol | Timolol |
How treatment is supplied | Oral solution or suspension | Solution or gel-forming solution |
Frequency of administration | BID to TID | BID |
Route of administration | Oral | Topical |
Time to onset of action | 2 weeks | 12 weeks |
Typical treatment duration | 9-12 months | 12 months+ |
Limitations | Monitoring at onset and dose changes | Not effective alone on exophytic or complicated IHs |
Side effects | Endocrine, neurologic, cardiac and respiratory complications | On intact skin, limited to mild skin irritation |
Contraindications | Severe asthma, significant cardiac abnormality, PHACES | Ulceration |
BID=twice daily; IHs=infantile hemangiomas; TID=three times a day.
Table 2. Treatment indications for IHs
| Oral propranolol (+/- systemic corticosteroid) | Topical timolol |
Ulceration/erosion | + | - |
Disfigurement (e.g. facial, diaper area/genital, female breast) | + | + |
PHACES/LUMBAR | +/- | + |
Rapid enlargement | + | - |
Obstruction of vital structure (e.g. periocular, airway, spine) | + | - |
Uncomplicated or minimally symptomatic mixed or deep IH | + | +/- |
Uncomplicated or minimally symptomatic superficial IH | + | + |
KEY: + (indicated); - (best avoided); +/- (applicable in select cases) | ||
IHs=infantile hemangiomas.
Oral corticosteroids still have a role and are now mostly used as adjunctive therapy to quickly arrest growth or relieve compression of vital structures, such as the airway, visual axis or spinal cord. Systemic corticosteroids are also useful for IHs involving the parotid gland, as these frequently do not respond as quickly to propranolol monotherapy. A short exposure to this drug class limits the formerly frequent side effects of hyperglycemia, hypertension, susceptibility to infection, irritability, gastrointestinal discomfort and growth suppression.
Given the reported consistency of response in a majority of cases, oral and topical beta blockers are an important addition to the physician's armamentarium aimed at the treatment of IHs.
