Immunisation for overseas travel
8.1 Introduction
Immunisation requirements for international travel are often the primary health concern of both prospective travellers and their doctors, usually followed by the choice of malaria tablet.
Immunisation is only one part of health advice for travellers. Attendance for immunisation provides an opportunity to deliver further health protection information on, for example, prevention of accidents and travellers' diarrhoea (Chapters 4 and 5), or specific advice relevant to the individual traveller.
The disease risk for the individual traveller should be assesed, as far as is possible, when choosing travel vaccines. The risk to a business traveller, for example, visiting only the most hygienic, air-conditioned premises for a few days should not be equated with that for someone travelling extensively to rural areas of the same country where not only is the risk to health increased but the facilities for medical treatment are likely to be less developed. The information on which to base such decisions is sometimes inadequate, not least because of limited reporting from some of the geographical areas of greatest risk. Some risks may be seasonal, or limited to certain geographical areas, and many are influenced by personal lifestyle or occupation, eg the risk for hepatitis B and HIV (Chapter 9). The risk of vaccine preventable disease for package holiday travel will depend on the itinerary and on the behaviour of the individuals involved, but will often be low.
Travellers may be informed by travel companies or embassies that ìno vaccinations/immunisations are neededî or ìnothing is neededî for a certain destination, and may omit to seek further medical advice. Education of travellers should include the information that ìnothing neededî may mean no certificates are officially required but that optional immunisations, usually more important for personal health protection, may be advised in addition to other health-related precautions.
8.2 International Certificates of Vaccination
The International Health Regulations adopted by the World Health Organization were devised to help prevent the international spread of diseases and, in the context of international travel, to do so with the minimum of inconvenience to the passenger (WHO, International Travel and Health 2000).
It should be remembered that the Regulations are more a public health measure for the receiving country than for protection of the individual.
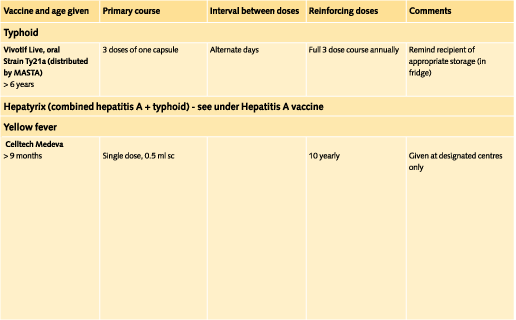
8.2.1 Yellow Fever
Yellow fever is now the only disease for which an international vaccination certificate may be required for entry into a country. Many countries (not the UK) require a valid International Certificate of Vaccination from travellers arriving from, or who have been in transit through, yellow fever infected areas or countries with infected areas. The maps inside the back page show the ìyellow fever endemic zonesî where there is a potential risk of infection. Some countries consider these zones as ìinfectedî areas for the purpose of International Certificate of Vaccination requirements. Other countries require a certificate from all entering travellers. Details of requirements are included in the entries for individual countries (Chapter 3). They are published annually in International Travel and Health, Vaccination Requirements and Health Advice (WHO). Failure to provide a valid certificate to the port health authorities could, in some circumstances, result in a traveller being immunised, denied entry or quarantined.
The International Certificate is valid for ten years beginning ten days after the vaccination date; this should be entered with the month written in letters. It should be signed by the person authorised by the national health administration (a stamp alone is not acceptable) and by the patient (or parent/guardian). (NB. All the partners in a practice which is a Yellow Fever Vaccination Centre are deemed by the Department of Health to be authorised persons). The manufacturer and batch number of the vaccine and the official stamp of the centre must also be included in the correct space provided.
If a physician advises that an individual should not be immunised on medical grounds, including infants under nine months of age, an exemption certificate may be provided (Appendix 1).
Yellow fever vaccination is recommended for travel to all countries in the endemic zones, whether or not an international certificate is required, and especially if rural areas will be visited. (See country by country advice).
8.2.2 Yellow Fever Designated Centres
Yellow fever vaccine may be administered only at centres which are designated by the national health administration and recorded with WHO. This is to ensure that vaccine storage, administration and certification is carried out correctly. (The current UK list of designated centres is available from http://tap.ccta.gov.uk/doh/yellcode. nsf/pages/Home?open, together with information for practices wishing to apply for designation.)
8.2.3 Cholera
In 1973, the International Health Regulations were amended so that no country should require a certificate of vaccination against cholera (WHO, International Travel & Health 1994). This followed acceptance that cholera vaccination does not prevent introduction of the infection into a country. Many countries continued to require proof of cholera immunisation long after 1973, but gradually the present position has been reached where there are no official requirements.
Until recently unofficial demands at a few international air and sea ports resulted in travellers continuing to request immunisation for certification. Reports of such incidents are now extremely rare, and appear to be confined to remote land borders in areas where there have been recent cholera outbreaks.
The conventional parenteral vaccine provided poor protection and is no longer available in the UK. In the rare circumstance where an unofficial demand may be anticipated, confirmation of non-requirement of cholera vaccine may be given on official notepaper signed and stamped by the medical practitioner (Appendix 1). Some new generation cholera vaccines are marketed in certain European countries.
Most travellers are at extremely low risk of contracting cholera. Prevention is by food and water hygiene (see Chapter 5).
8.2.4 Meningococcal vaccination for the pilgrimage to Mecca
Saudi Arabia requires pilgrims to produce proof of immunisation against meningococcal infection issued not more than three years and not less than ten days before arrival in the country. Details are listed in the Saudi Arabia entry (see also important information at 8.4.4).
8.3 Vaccines
| Live vaccines | Inactivated vaccines | ||
| Measles | and MMR | Diphtheria toxoid | and combination vaccines |
| Mumps | and MMR | Tetanus toxoid | and combination vaccines |
| Rubella | and MMR | Pertussis | and combination vaccines |
| Oral poliomyelitis | Poliomyelitis (injectable) | ||
| Oral typhoid | Haemophilus influenza b (Hib) | ||
| BCG (TB) | Influenza | ||
| Yellow fever | Pneumococcal | ||
| Hepatitis A | and combination vaccines | ||
| Hepatitis B | and combination vaccines | ||
| Typhoid Injectable (and hepatitis A combined vaccine) | |||
| Meningococcal (A&C) | |||
| Japanese encephalitis | |||
| Tick-borne encephalitis | |||
| Rabies |
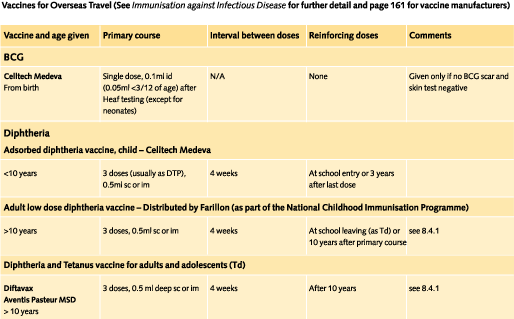
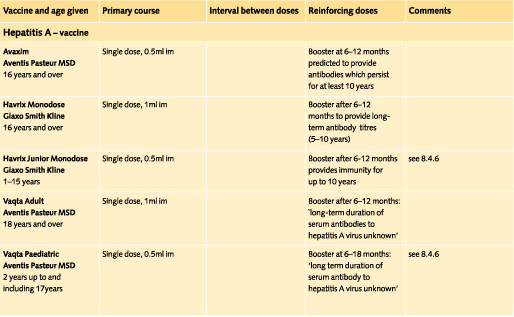
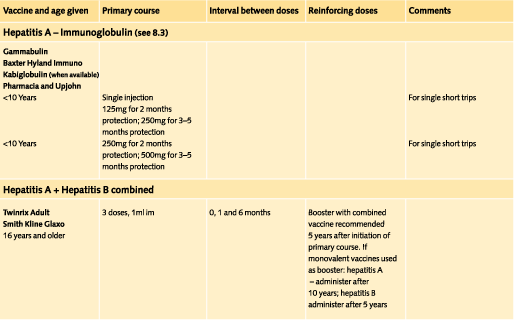
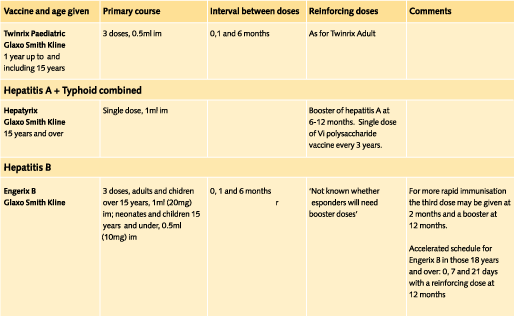
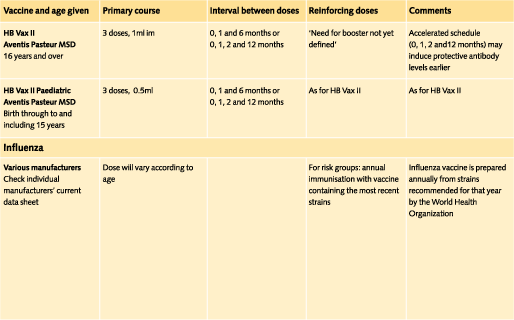
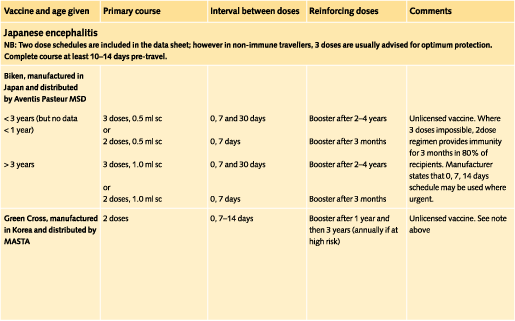
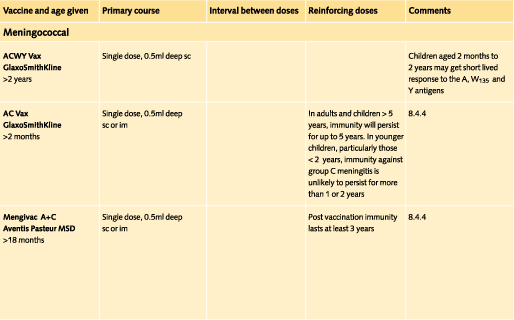
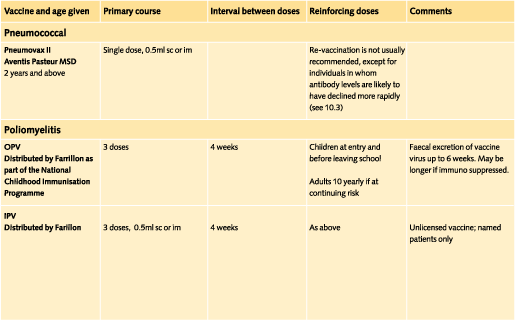
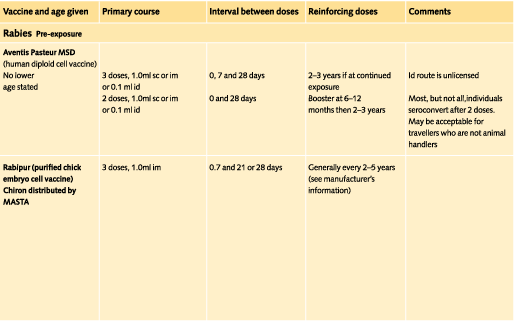
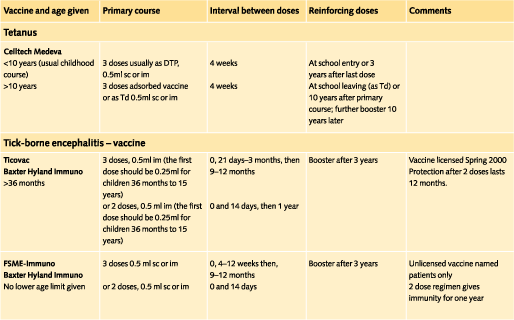
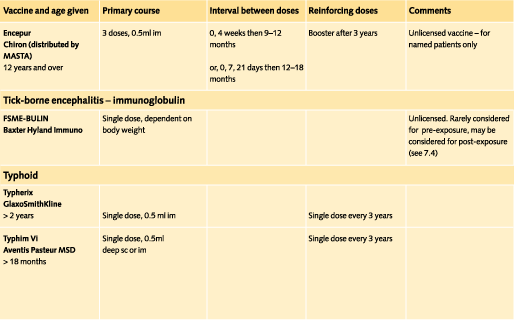
Doses and recommended schedules are summarised on pages 97 to 108. Information about individual vaccines is contained in the current edition of the memorandum Immunisation against Infectious Disease.
8.4 Recommendations
These are contained in the invidual country entries in Chapter 3. They assume that childhood immunisations, including BCG, are up to date.
8.4.1 Routine immunisations
All individuals should have completed primary tetanus, diphtheria and poliomyelitis courses. A full course comprises five doses of each. When over ten years has elapsed since the primary course and travel is to a developing area a tetanus booster should be given; a diptheria booster should also be given if travel is for more than one month. The appropriate combined diptheria/tetanus preparation is now normally used when either of these is due. A polio booster may be advised for travel to certain countries if ten years has elapsed since the primary course (see country by country advice).
8.4.2 Influenza and pneumococcal vaccines
Those who are recommended to have influenza or pneumococcal vaccine as part of UK policy are advised to be immunised before travel.
8.4.3 Hepatitis A
Where hepatitis A protection is recommended for travel, vaccine is the preferred option rather than normal immunoglobulin. There is some evidence of protection even when vaccine is given after exposure, so that if time before departure is short, the vaccine is still considered likely to prevent or at least modify the infection.
8.4.4 Meningococcal vaccine
Conjugate meningococcal C vaccine (MenC) has recently been introduced into the routine UK childhood immunisation programme. This vaccine protects only against group C meningococcal infection, while much meningococcal infection abroad is caused by Group A. The currently used vaccine for travel is therefore meningococcal A&C polysaccharide vaccine.
A quadravalent vaccine, also containing Y and W135 strains, is now more widely available and is the recommended vaccine for all pilgrims to Saudi Arabia.
Some mild urticarial reactions have been reported in children given A&C vaccine shortly after MenC vaccine. It is not known whether this rate is higher than could be expected with A&C alone, but an interval of two weeks is recommended if A&C vaccine is required following MenC. Until further evidence emerges it is also currently recommended that where MenC vaccine is due following A&C vaccine, the MenC vaccine is delayed until six months after A&C vaccine. In high risk situations, however, MenC vaccine should not be delayed. The local Consultant in Communicable Disease Control or Immunisation Co-ordinator should be consulted.
8.4.5 Combination vaccines
Combination travel vaccines are now available containing more than one vaccine in one preparation, such as adult diphtheria and tetanus. Vaccines recommended should be appropriate for the individual. Where a recipient requires protection against both diseases, at least for the early doses, a combination preparation can be useful.
However, where the two components of a combination (eg hepatitis A with hepatitis B or hepatitis A with typhoid) are not both indicated for the individual traveller, the combined vaccine should not replace the individual vaccines. Where the individual components differ in duration of immunity or number of doses required to complete the course, combined vaccines can also complicate scheduling. Single antigen vaccines may be required for boosters.
Modern vaccines and sharp needles produce little discomfort when skilfully administered and many recipients are unable to report the exact number of injections received.
8.4.6 Infants and small children travelling
Routine infant immunisations may be advised earlier than normally scheduled when children are travelling to high-risk countries for prolonged periods and may have close contact with the indigenous population (for example staying with relatives abroad). In particular, earlier immunisation may be advised if travel is so prolonged that routine childhood immunisations would be delayed.
Hepatitis B vaccine and BCG can be given from birth where indicated. Polio can, if necessary, be commenced from birth, but an extra dose is then advised later on; DTP-Hib can be administered from six weeks of age. Children over six months of age who have not yet received their first dose of MMR, travelling to visit relatives in a measles endemic area, should be offered MMR. However two further doses of MMR are then recommended: one as soon as practicable after the first birthday and the normal pre school booster.
Hepatitis A is usually a mild disease in young children, and infection results in lifelong immunity. Vaccine is therefore often considered unnecessary in this age group (although opinions differ). It is more likely to be considered for those travelling to visit friends and relatives for longer periods in areas of high endemicity. There is an argument that the children should be immunised to prevent secondary infection in non-immune adult contacts of the children, eg play group leaders, on their return.
The addition of conjugate meningococcal group C vaccine (MenC) to the routine schedule may result in a small child travelling to, for example, Africa requiring the A&C vaccine close to the new vaccine (see 8.4.4).
The table of immunisations (pages 94-104) provides the lower age limit for travel vaccines where these are specified and the varying ages at which the paediatric dose changes to the adult dose.
8.5 Schedules
Wherever possible, the recommended intervals between doses and between vaccines should be followed and time allowed for antibody to be produced, courses completed and any reaction to have dissipated before the date of travel.
In theory each travel vaccine should be given at least ten days (and preferably three weeks) from another in order to identify the source of any reaction. In practice, time constraints, travel dates and sheer practicality have resulted in many vaccines being given simultaneously without apparent adverse effects.
8.5.1 Live Vaccines
Live vaccines should be administered at least three weeks apart or on the same day. However, the two oral vaccines, typhoid and polio, are usually separated (by at least two weeks) on the theoretical grounds of possible interference in the gut. There is no evidence to preclude oral typhoid being given with yellow fever or human normal immunoglobulin (HNIG).
Live virus vaccines may suppress the tuberculin test and so should be delayed until after the test has been read.
8.5.2 Inactivated Vaccines
Inactivated vaccines can be given simultaneously with any other vaccine, but at a different site, the number given taking into account the comfort of the patient. Concurrent administration of vaccines can make it difficult to elucidate adverse reactions. An exception to the simultaneous administration rule concerns meningococcal A&C and the recently introduced conjugate meningococcal C vaccine (see Meningococcal vaccine 8.4.4).
8.5.3 Human Normal Immunoglobulin (see 8.4.3)
The antibody response to MMR (or measles, mumps or rubella given separately) could be inhibited by HNIG which should be delayed until three weeks after the vaccine. If HNIG has already been given, three months should elapse before giving MMR.
HNIG has not been shown to inhibit yellow fever, oral typhoid or BCG and any effect it has on OPV is unlikely to be significant where the OPV is a booster.
HNIG is anyway usually given after the vaccines and closer to the departure date because of its rapid efficacy and shorter duration of action.
8.5.4 Timing
Courses of most travel vaccines, plus the single dose vaccines, can be administered over a four week period. The final doses should ideally be completed a little ahead of the departure date to allow immunity to develop. It can take up to four weeks, for instance, for full immunity to develop following Japanese encephalitis vaccine. (This vaccine is anyway recommended to be completed at least ten, and preferably 14, days prior to travel because of the possibility of a delayed allergic reaction.)
More time will be required if a primary course of tetanus, polio or diphtheria is necessary. If the full course cannot be completed before departure, it is usually worth giving the maximum number of doses that the travel departure date allows, completing the course on return.
Travellers should be encouraged to plan to start immunisations well in advance of travel.