Green Tree Frog
Litoria caerulea | |
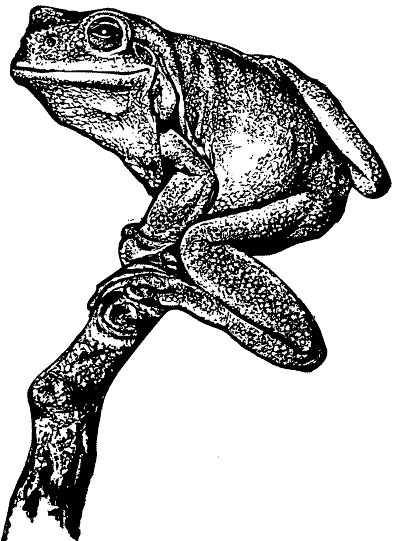
| Found in a wide variety of habitats, including human dwellings, this is one of Australia's largest and most common frogs. All frog species have sensitive skins which are protected by a moist coating containing many chemicals. This tree frog secretes the chemical Caerulein, now artificially produced as a drug for use in human medicine. | 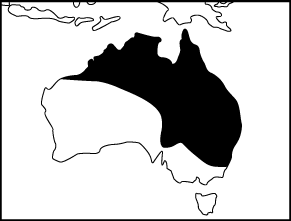 |
Authority: White, 1790
Description (Clyne 1969; Tyler et al. 1983; Cogger 1992; Barker et al. 1995; Van Dyck 1997):
The dorsal surface is smooth and bright green to olive in colour. The posterior portion of the head contains large glands with many tiny pores. The ventral surface is white and coarsely glandular. Some individuals contain an irregular white stripe from the corner of the mouth to the shoulder. Many specimens have small distinct white spots on their back, sides and limbs. Fingers and toes are long with large terminal discs. Fingers are approximately one third webbed and toes nearly three- quarters webbed. The second finger is longer then the first. The colouration of the thighs varies from yellow to maroon.
There is a large fleshy fold of skin that overhangs the distinct tympanum and the vomerine teeth are between the choanae. They contain large inner and outer metatarsel tubercles.
Males often have a green tinge on the throat region. Males are 66-77mm in length; females 60- 110mm. The mating call resembles a deep continuous “crawk”.
Similar species (Barker et al. 1995):
L. gilleni and L. splendida have enlarged head glands as in L. caerulea. However L. gilleni tends to be smaller in size. It also appears to replace the distribution of L. caerulea around the Alice Springs region. L. splendida, unlike L. caerulea has skin glands that cover the entire head region. L. cavernicola also resembles L. caerulea however they lack the large fold of skin that overhangs the tympanum. The vomerine teeth of L. cavernicola are behind the choanae rather then in between as in L. caerulea.
Range (Clyne 1969; Cogger 1992):
Commonly found from coastal to arid habitats in north-western W.A., north-eastern S.A., New South Wales down to about Sydney, throughout Queensland and most of NT. Also found in Southern Papua New Guinea.
Ecology and behaviour (Harrison 1922; Cogger 1992; Barker et al. 1995; Van Dyck 1997; Williams et al. 1998):
The green tree frog is one of the most commonly encountered frogs is Australia. It has a habit of occupying lavatories, letterboxes, water tanks, down pipes and other objects that are associated with human dwellings. When L. caerulea isn’t inhabiting kitchen cupboards or toilet bowls they are found hiding within heavy- foliaged creepers and similar vegetation, which is usually a considerable distance from water.
During the day they are relatively inactive, conserving water loss through evaporation. Not unlike other species they forage at night. L. caerulea are opportunistic predators of arthropods, small birds, rodents, bats, frogs, and just about anything else living that can be swallowed. The skin secretions of L. caerulea have been found to be lethal to a number of blowflies, whereby acting as a natural insect repellent.
Breeding biology (Harrison 1922; Tyler et al. 1983; Tyler 1985; Tyler 1994; Van Dyck 1997):
The reproductive biology of L. caerulea is similar to a number of other hylid species. L. caerula does not form large breeding choruses, but individual males call from exposed, elevated surfaces (e.g. branches of trees, fences). Calls only occur at night and commence early in October and are continued until March. The breeding season occurs from November to February, whereby females are attracted to certain calling males.
Breeding takes place in static, ephemeral waters where the water temperature is between 28 and 38 C and the depth of the water is between 5 and 50 cm deep. The eggs are not associated with any sort of parental care, but are directly laid on the surface of the water.
The spawn consists of about 200- 2000 brown eggs, which sink to the floor approximately 24 hours after being laid. The eggs contain two jelly envelopes and have a mean capsule diameter of 2.5mm. The mean ovum diameter is 1.3mm. The eggs hatch out in about 3 days and have reached the 18th stage. Tadpoles attain a mean length of 8.1mm by stage 24. A period of maximum growth follows, the embryo reaching 28.5mm by stage 29.
Maximum size is reached at stage 41 (41.0mm- 100mm). Metamorphosis occurs two months after hatching in the field. However under laboratory conditions, metamorphosis occurs three months after hatching. Tadpoles are of the typical lentic Litoria type. The tooth row formula is 2/3 and is well developed. The tadpoles are mottled brown with pigmentation increasing as development progresses. The ventral surface of tadpoles is initially dark in colour, but becomes progressively lighter with development. Development is completed in about six weeks. Adults have lived to an age of 23 years in captivity.
Literature cited:
- Barker, J., Grigg, G. and Tyler, M. 1995. A Field Guide To Australian Frogs. Surrey Beatty and Sons, Chipping Norton, NSW.
- Clyne, D. 1969. Australian Frogs. Lansdowne Press, Melbourne.
- Cogger, H.G. 1992. Reptiles and Amphibians of Australia. Reed Books, Chatswood, NSW.
- Tyler, M.J., Crook, G.A., Davies, M. 1983. Reproductive biology of the frogs of the Magela creek system, Northern Territory. Records of the South Australian Museum 18: 415-440.
- Tyler, M.J. 1985. Reproductive modes in Australian Amphibia In Grigg, G., Shine, G., and Ehmann, H. (eds), Biology of Australasian Frogs and Reptiles. Surrey Beatty and Sons, Chipping Norton, NSW.
- Tyler, M.J. 1994. Australian Frogs: A Natural History. Reed Books, Chatswood, NSW.
- Van Dyck, S. 1997. Raising a glass to the loo lord. Nature Australia Spring: 18-19.
- Williams, C.R., Wallman, J.F. and Tyler, M.J. 1998. Toxicity of green tree frog (Litoria caerulea) skin secretion to the blowflies Calliphora stygia (fabricius) and Lucilia cuprina (Wiedemann) (Diptera: Calliphoridae). Australian Journal of Entomology 37: 85-89.
