Spider neurotoxins
Polyamines
Despite the vast majority of spiders being harmless to humans, many spider venoms contain in their venoms potent small molecules called polyamines. Polyamines are often very effective and specific blockers of ion channels or of receptors. Their target receptors are those which recognize excitatory amino acids in the mammalian central nervous system and are classified into three major subtypes, ones which prefer N-methyl-D-aspartate (NMDA), quisqualate (QA), or kainate (KA) as type agonists respectively .
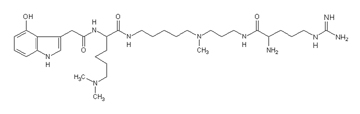
Argiotoxin, a glutamate-activated postsynaptic ion channel blocking polyamine from the spider Argiope lobata.
Spider toxins have been isolated which are selective and reversible noncompetitive antagonists of NMDA (N-methyl-D-aspartate) receptors in the mammalian brain. By way of example, Dolomedes okefinokensii (Fishing spider) bites rarely cause anything but the mildest of edema and possibly minor necrosis. The venom, however, has yielded a compound that may have use as an analgesic, a polyamine that reversibly blocks L-and R-type voltage-sensitive calcium channels.
Other examples of the usefulness of spider polyamines are the alpha-agatoxins Aga-GI and Aga-G2 from the American funnel web spider Agelenopsis aperta. These low molecular weight molecules cause rapid, reversible paralysis correlated with use-dependent postsynaptic block of excitatory postsynaptic potential and ionophoretic glutamate potentials. Aga-GI is a toxin capable of inhibiting an elusive neuronal calcium channel that is resistant to blockage by either omega-conotoxin GVIA or nifedipine making this component a novel tool with which to probe this channel. Intravenous or intracerebroventricular administration of Aga-G2 produces dose-dependent suppression of behavioral convulsions induced in rats by kainic acid, picrotoxin, or bicuculline. The combination of their small size but potent actions makes these toxins a notable class of compound worthy of in-depth study.
Another interesting toxin quite useful in studying receptors for excitatory amino acids in the mammalian central nervous system is jorotoxin from the venom of Nephila clavata (Joro spider). Jorotoxin has been shown to be a specific blocker of the postsynaptic glutamate receptors, in contrast with bacterial pertuissus toxin that blocks the presynaptic glutamate receptors. These low molecular weight toxins may provide novel tools for anticonvulsant research and therapy, demonstrating yet again the potential use of venom components to be used as investigational ligands or even therapeutics in their own right.
Spider venom peptides
Mu-Agatoxins
The nomenclature of spider peptidic neurotoxins is, unlike that of scorpions, based on the same convention as that of cone-snails and snakes: alpha-toxins inhibit the acetylcholine receptor; mu-toxins directly abolish muscle action potentials through the inhibition of muscle sodium channels; and omega-toxins prevent voltage-activated entry of calcium into the nerve terminal and the release of acetylcholine.
Spider mu-toxins are cysteine-rich polypeptides, which cause irreversible paralysis and repetitive action potentials originating in presynaptic axons or nerve terminals. Clinical effects of a bite from one of the North American funnel web-spider Agelenopsis aperta are negligible. The venom, however, has yielded several interesting peptides that have pharmacological properties that may prove to be quite useful. Two specific mu-agatoxins have been identified from A. aperta, mu-agatoxin-I and mu-agatoxin-IV, both of which contain 36 amino acids and four internal disulfide bonds. Although these toxins specifically modify voltage-sensitive sodium channel activity, they have structural similarities with a plethora of other peptide toxins targeting a myriad of channel types. Analysis of these variations may shed light not just on functional residues of the particular scaffold but also provide data as to the structure and specificity of the binding sites of the channel being affected. This adds to the emerging wealth of data of a common scaffold being modified for divergent use.
The omega-agatoxins from A. aperta consist of two subtypes of neuronal calcium channel toxins with different structural characteristics and binding specificities. Type I agatoxins, such as omega-Aga-I, may define a binding site on neuronal calcium channels that is common to both vertebrates and invertebrates. Type II omega-toxins, such omega-Aga-II, III and IV, share limited amino acid sequence similarity with Type I toxins. However, omega-Aga-IVA is able to block omega-CTX-GVIA (Conus geographus) resistant calcium channels. This use of structurally distinct toxins from common as well as divergent sources is an excellent manner in which to study a channel.
The peptidic neurotoxins from A. aperta have similar locations of cysteine residues with neurotoxins from the venom of another North American funnel web spider Hololena curta as well as neurotoxins from the more distantly related Brazilian spider Phoneutria nigriventer (Wandering spider). This conservation of structure among these three species is interesting to view from a taxonomic standpoint in that venom peptide sequences may be useful as chemotaxonomic tools. Despite being the only lethal species amongst the three spiders, the toxins of the Phoneutria nigriventer share strong structural similarity, particularly in the location of the cysteine residues with neurotoxins from these other two spiders.
While the lethal neurotoxin PhTx1 from Phoneutria nigriventer venom has a primary structure shows no homology to any other identified spider toxin, cDNA libraries constructed from the venom glands revealed that the structure of the preprotoxin initially synthesized by the Tx1 gene shows similarity in sequence and also in processing with the synthesis and processing of omega-agatoxin IA from A. aperta. Thus, this toxin may not be quite so unrelated but rather may represent a case of a spider that is taxonomically divergent, as P. nigriventer is from the more closely related two N. American species, with the venom diverging along with it.
Delta Atracotoxin
Unlike the American funnel web spiders, the venoms of Australian funnel web spiders are quite lethal, consisting of a large number of acute acting neurotoxins. These venoms slow the inactivation of primate sodium channels causing envenomation symptoms involving pain at the bite site, salivation, lachrymation, piloerection, generalized skeletal muscle fasciculation, sweating, nausea, vomiting, diarrhea, pulmonary edema, dyspnoea followed by respiratory failure, tachycardia and hypertension followed by hypotension and circulatory failure.
The primary toxic components of the venoms of two species have been isolated and characterized. The lethal toxins from A. robustus and H. versuta venoms are the 42 amino acid peptide components robustoxin (atracotoxin) and versutoxin respectively. Versutoxin differs from robustoxin by only 8 amino acid residues. Disulfide-bridged cysteine residues at both the amino- and carboxy-termini and a triplet of cysteines at residues 14-16 makes these components unprecedented amongst neurotoxins.
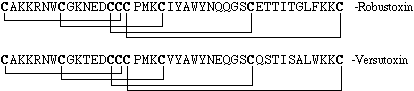
Comparison of rubustoxin and versutoxin.
These presynaptic neurotoxins produce spontaneous, repetitive firing of autonomic and motor neuron action potentials. Characteristic of this autonomic storm is a wave of endogenous acetylcholine, noradrenaline and adrenaline. These two toxins bind to tetrodotoxin-sensitive sodium channels, competing with alpha-scorpion toxins. These toxins cause a voltage dependent slowing of sodium channel inactivation by binding to the outer surface of the channel, thus interfering with the conformational changes necessary for gating of the channel.
Latrotoxin
In contrast to the mostly channel binding small peptide toxins from the Australian funnel web spiders and that from most other spiders, the major bioactive component (latrotoxin) from the venom of the lethal American black widow spider (Latrodectus mactans) is a much larger protein. Latrotoxin is responsible for many of the neurological symptoms clinically seen, causing a massive release of acetylcholine that is stimulated by the presence of Ca2+ ions. It is interesting to compare the activity of latrotoxin with the smaller peptidic venom component omega-conotoxin. Latrotoxin is an activator of synaptosomal calcium uptake, while omega-conotoxin GVIA is an inhibitor of voltage-sensitive calcium channels of the N-type, yet both toxins ultimately produce cramping or rigid paralysis.
