Visually Track Skin-derived Immune Cells
Introduction
The skin has been considered to be immunologically important in the area of skin-associated lymphoid tissue (SALT) since various kinds of immune cells such as dendritic cells (DCs), T cells, and mast cells reside in the skin, and furthermore, many dynamic immunological reactions occur at the site of the skin. Of note is a recent novel finding that normal skin contains twice as many T cells that are present in the blood circulation, which can initiate and perpetuate immune reactions in the absence of T-cell recruitment from the blood. However, it is very difficult to identify immune cells that originated from the skin and to know the fate of skin-derived T cells. Additionally, there has been no direct evidence of T-cell migration from the skin to draining lymph nodes (LNs) (Figure 1).
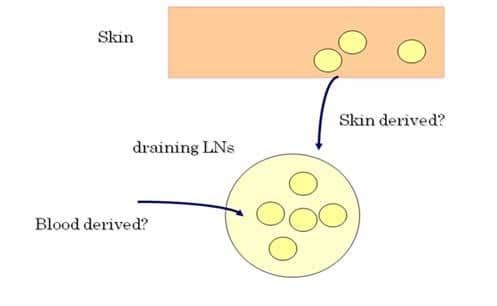
In order to answer this largely unknown question, we have utilized Kaede-transgenic (Tg) mice and demonstrated the trafficking of T cells between the skin and LNs in vivo. We have also investigated the important role of skin-derived regulatory T cells (Tregs) to terminate the contact dermatitis.
What are Kaede-transgenic mice?
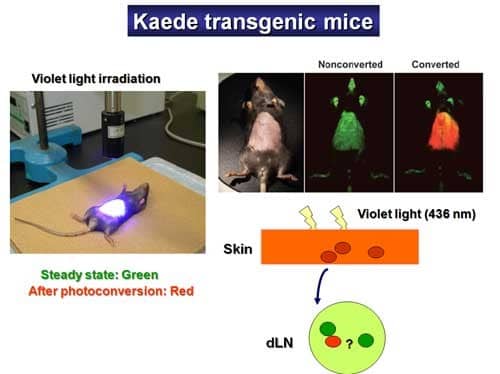
"Kaede" refers to the Japanese maple leaves that turn their color from green to red in autumn. Kaede protein is a newly developed photo-convertible fluorescent protein that can change emission spectra in response to violet light exposure (436 nm). In Kaede-Tg mice, all cell types constitutively exhibit Kaede-green fluorescent signals, and immediately after violet light exposure to the skin, cells in the exposed area began to emit Kaede-red fluorescent signals, thus, labeling in vivo skin-derived cells under physiological conditions (Figure 2).
By using this Kaede-Tg mouse, T-cell migration was visually demonstrated even in the steady-state from the skin to draining LNs in mice. Although the number is very small, skin-derived T cells and DCs are found in skin-draining LNs, suggesting a constant communication between the skin and LNs (Figure 3).
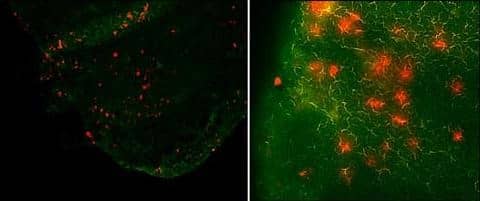
Footprints of skin-derived T cells
How about the T-cell dynamics in the inflamed state? Kaede-Tg mice are sensitized with hapten at the dorsal skin and are challenged at the abdominal wall skin with the same hapten and then exposed to violet light. The number of Kaede-red cells in the draining LNs was increased by almost 10 times over steady state, suggesting the accumulation of memory T cells into the abdominal skin. Of great surprise was the finding that Kaede-red T cells were observed both in the blood circulation and the ear skin after re-challenge, indicating that some of the skin-derived T cells in draining LNs re-enter the blood circulation and remigrate into the skin in response to hapten rechallenge.
The role of skin-derived Tregs in contact dermatitis
Following our interest in the Treg functions, we asked how skin-derived Tregs work on contact dermatitis. To this end, we have generated Foxp3 reporter mice expressing human CD2 and human CD52 chimeric protein (Foxp3hCD2/hCD52 mice). Since Foxp3+ cells co-express hCD2 on the cellular surface in these mice, live Foxp3+ Tregs are sorted with anti-hCD2 mAb and depleted with neutralizing antihCD52 Ab. We found that depletion of Tregs in the elicitation phase caused enhanced and prolonged ear swelling response compared with the control, suggesting that Tregs are responsible for terminating skin inflammation in contact dermatitis. Since Tregs from the skin showed significantly higher mRNA expression of T-cell suppression-associated molecules such as IL-10 and TGF-beta, and furthermore, skin-derived Tregs exhibited significantly stronger suppressive activity both in vivo and in vitro, it is suggested that skin-derived Tregs that traffic to draining LNs and then recirculates back to the skin, contribute to the downregulation of cutaneous immune responses leading to the termination of contact dermatitis (Figure 4).
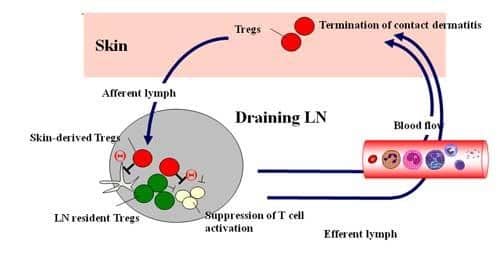
Conclusion: Skin is an important peripheral lymphoid organ
We have uncovered a unique ability of Tregs to migrate back and forth between the skin and LNs, which now form a new framework for our understanding of Treg homing. From a clinical perspective, the precise mechanism by which Tregs work in the elicitation phase is an important issue to be addressed, which will give us important clues supporting the development of innovative therapeutic approaches for contact dermatitis. Also, these studies are evocative of the classic concept of SALT and underscore the critical role of skin as a peripheral lymphoid organ, because the classic concept of SALT simply defines the skin as a non-lymphoid organ that is only functionally connected with the draining LNs, at least under homeostatic conditions. However, our studies have revealed that T cells that migrate to the skin can egress and return to skin-draining LNs, and even further, circulate in the blood as well as other tissues. These findings refresh the concept of SALT with the consequence that, even under the homeostatic condition, skin is an active organ of the immune system. Thus, the skin should be recognized as an important peripheral lymphoid organ.
